Thanks for obtaining approval to recruit to the GenOMICC study! This virtual site visit describes the key next steps you need to take in order to get started with recruitment.
The GenOMICC study aims to recruit eligible patients within the ICU/HDU, PICU or NICU environments. Recruitment is relatively straightforward and involves screening, consent, a single DNA sample and completion of an online CRF.
Before recruitment can begin, local R&D approval should be in place and members of the research team appropriately trained to conduct clinical research with patients in your area (i.e., GCP trained).
We do not provide a GenOMICC specific investigator site file and ask sites to set up and maintain their own GCP compliant file. Our sponsor’s website has guidance on setting up and maintaining an investigator file, including an index of the documents required. Please refer to the SOP CR001 here - ACCORD
The site file must only be accessed by authorised staff and stored securely.
No screening log is required, and we do not need to receive copies of any delegation logs. Documents held electronically are acceptable.
The current approved study documents can be found on our website here - Study documents
Use the documents relevant for your area.
You can find a full list of the eligibility requirements here - Entry
We encourage recruitment at the earliest opportunity, however, there is no time limit from admission to ICU/PICU/NICU and patients can be recruited at any time after they become eligible, even if they are stepped down to the ward.
All consenting patients can be included and there is no upper or lower age limit.
Exclusion - There is only one exclusion criterion and that is, please exclude any patients who have had a bone marrow transplant.
Participant enrolment procedures are covered extensively in our protocol. Consent can only be undertaken by members of staff trained in consent procedures and who hold GCP certification.
Patients should always be approached for consent directly in the first instance. If a patient is unable to consent for themselves then we must follow the appropriate legislation in place for your area.
Scotland only
In line with the Adults with Incapacity (Scotland) Act, those who are able to act as a legal representative to give consent on behalf of an adult without capacity to do so themselves are:
The adult’s Welfare Guardian or Welfare Attorney and if not appointed, the adult’s nearest relative.
Rest of the UK
In line with the Mental Capacity Act, advice from a consultee should be sought on whether an adult lacking capacity to consent would wish to be included in our research. Consultees are not asked to give consent on behalf of the adult, but rather to provide an opinion on the views and feelings of the potential participant.
Consultees can be approached as:
Personal consultees; that is a person who cares for the adult lacking capacity or is interested in that person’s welfare, but is not doing so for remuneration or acting in a professional capacity (i.e., nearest relative or close person)
If a personal consultee is not available or unwilling to give advice then a nominated consultee (i.e. a professional who is independent of the study) can do so. If this option is utilised as a last stage option, seek advice from the patients personal consultee if they become available as soon as possible, even if the patient is expected to make a full recovery and be able to consent for themselves.
If a patient regains the capacity to consent for themselves, they should be approached at an appropriate time and given the opportunity to do this. The study protocol covers this aspect of consent in detail.
Consent forms are retained at site, in a secure area or locked filing cabinet where only authorised staff have access.
We have separate GenOMICC specific deferred consent guidance.
In England, Wales and Northern Ireland, a deferred consent process can be applied in some instances. In these cases a sample will be taken in line with our recruitment procedures from any patient meeting the entry criteria. The sample can be held at the recruiting site for up to 7 days pending appropriate consent / advice. No samples will be transferred to the central laboratory for testing, or stored at site beyond 7 days, without consent having first been obtained.
The option of deferred consent is not currently available in Scotland.
REDCap is the online database used to complete patient clinical Case Report Forms electronically (eCRFs).
Training guides and further information is held on our website here: REDCap
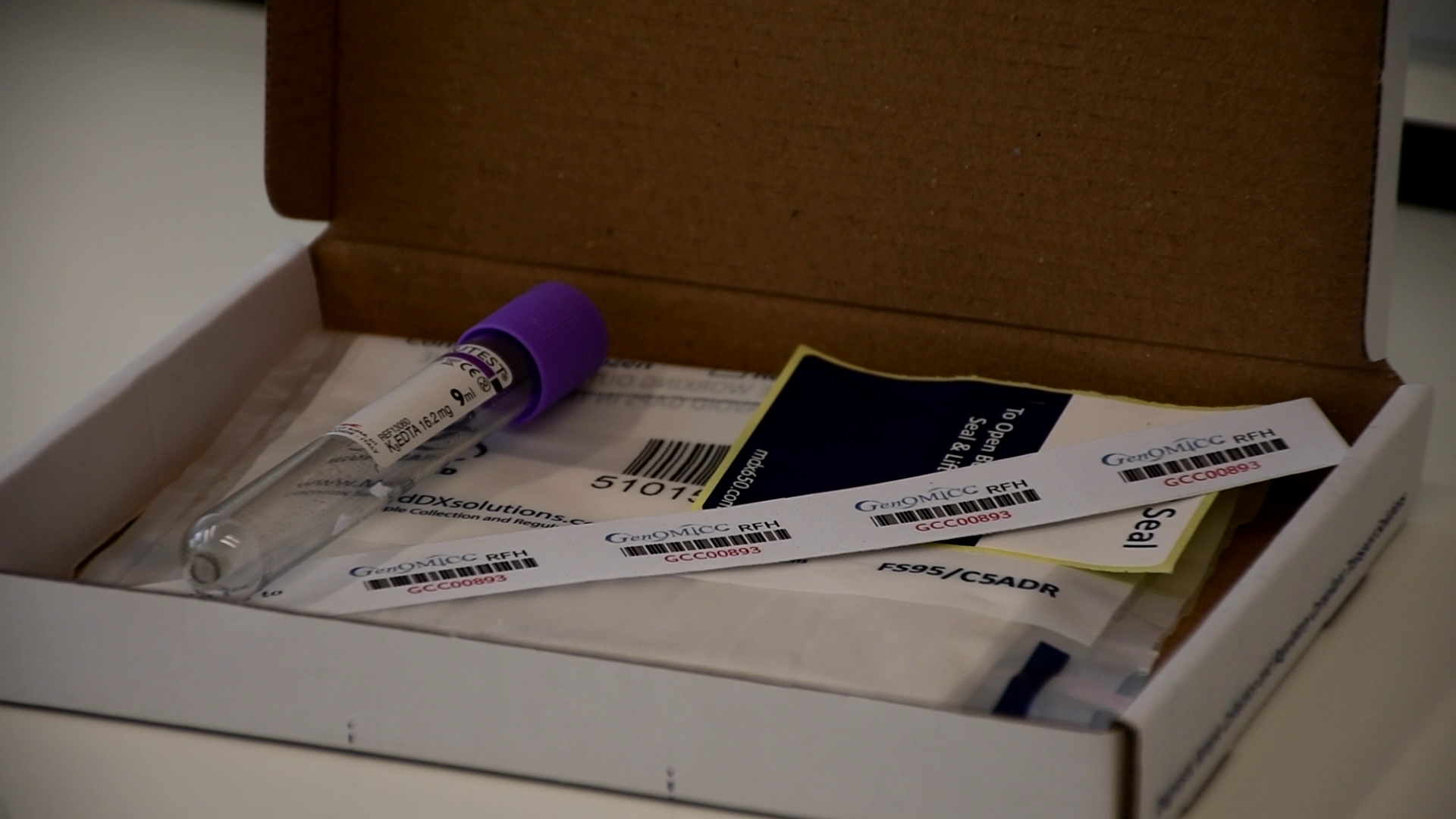
Samples are returned to us in the postage paid UN3373 specimen boxes that we provide. No sample processing of any kind is required at site. Samples should be returned to us as soon as possible after they have been taken and will be fine working through the postal system for a few days.
Each specimen box contains an EDTA tube and a strip of barcodes (4 stickers). Use one for the consent form and the rest for the EDTA tube(s). Discard any that are unused. Only one strip to be used per patient. It is this same number that is used to create the eCRF record on REDCap.
We supply specimen kits in multiples of 12 and can supply as many as you can store. Additional specimen boxes can be requested from: genomicc@roslin.ed.ac.uk
More details on sampling can be found on our recruitment procedures page
We encourage co-enrolment with all other studies including interventional trials. No further paperwork is needed to co-enrol patients to GenOMICC. Checks only need to be made with the other study.
Participants are free to withdraw from this study at any time without giving reason and without detriment to their medical care. If a participant changes their mind and doesn’t want to continue to take part, let us know and we’ll advise next steps.
We are approved to recruit patients retrospectively, that is, after discharge if they were not recruited when in hospital. We ask sites to obtain consent, complete the eCRF and send out sampling kits and we take care of the rest. If you have resources to consider this aspect of recruitment, please let us know and we’ll talk you through this process and send our additional guidance.
The GenOMICC team are available by email, telephone and online virtually via Zoom or Teams to provide team training or one-to-one calls.
In summary, before you start, you should: