
GenOMICC has expanded its recruitment cohort to include babies born before 32 weeks postmenstrual age.
The aim is to identify genetic variants that contribute to an increased likelihood of developing chronic conditions such as Bronchopulmonary Dysplasia (BPD) in the UK population.
With as many as 2000 new BPD cases occurring in the UK each year, there is an urgent need to find new treatments.
Please contact us if you are interested in setting up recruitment in your area and we can help.
Recruitment is designed to be as straightforward as possible involving consent, a single sample and completion of a short case report form.
Our virtual site page provides an overview of GenOMICC and key information on setting up in your area: Virtual site visit
All babies born less than 32 weeks postmenstrual age for any reason.
Whilst early sampling is desirable, there is no time limit for recruitment. Multiple sample options are possible and the central GenOMICC study team can supply specimen kits and all their component parts.
We have a parent and guardian consent form and information sheet to record consent available online: Study documents
In some cases antenatal consent may be obtained if appropriate and an early birth is expected.
Anyone approaching parents and caregivers for consent must have completed GCP training.
One of the following sample types should be obtained:
Whole blood in EDTA tube – preferred option in all cases: 0.5-4kg: 0.5mls
Umbilical cord blood in an EDTA tube: 1ml
Dried blood spots: >0.5kg: 4 spots collecting 0.5ml
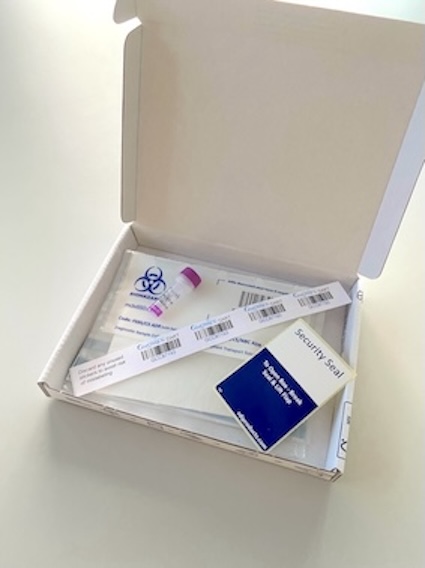
We provide biologically approved packaging for returning samples. The specimen kits are already pre-labelled with a postage paid return address and are issued in multiples of 12. We supply a 0.5ml EDTA microtainer tube inside each specimen kit as standard.
We can send a separate supply of dried blood spot cards and /or 1ml EDTA tubes (that could be used for cord blood) if alternative sampling methods are preferred. All samples are returned in the same way, within the specimen kits that we send.
Take all four of the of GenOMICC barcoded stickers (called GCC IDs) from inside one of the return GenOMICC specimen boxes that we have sent to you.
Label the blood tube or dried blood spot card with one of our GCC ID barcoded stickers and also add an additional barcode to the outside of the plastic sample bag with absorbent material. Seal the bag and package into the GenOMICC specimen box and return to the Wellcome Trust Clinical Reseach Facility (WTCRF), Edinburgh as soon as possible.
Please send samples as soon as possible after they have been taken. They will be stable for several days at ambient temperature so there is no problem posting samples on a Friday, over the weekend or during public holidays.
We have separate guidance for sampling using dried blood spot cards.
Use one of the GenOMICC barcode stickers to label the consent form. The participant consent form, REDCap record and blood sample must all be created and labelled with the same GCC Number.
This means we can easily reconcile samples received at the lab, with the correct eCRF.
Destroy any unused stickers - the same number should never be used twice.
Store the consent form in the local site file, which should only be accessible by authorised staff, in a secure area.
A minimal electronic case report form will be completed on REDCap, where our dedicated GenOMICC team can provide access and support.
Our REDCap guidance can be found online: REDCap guidance
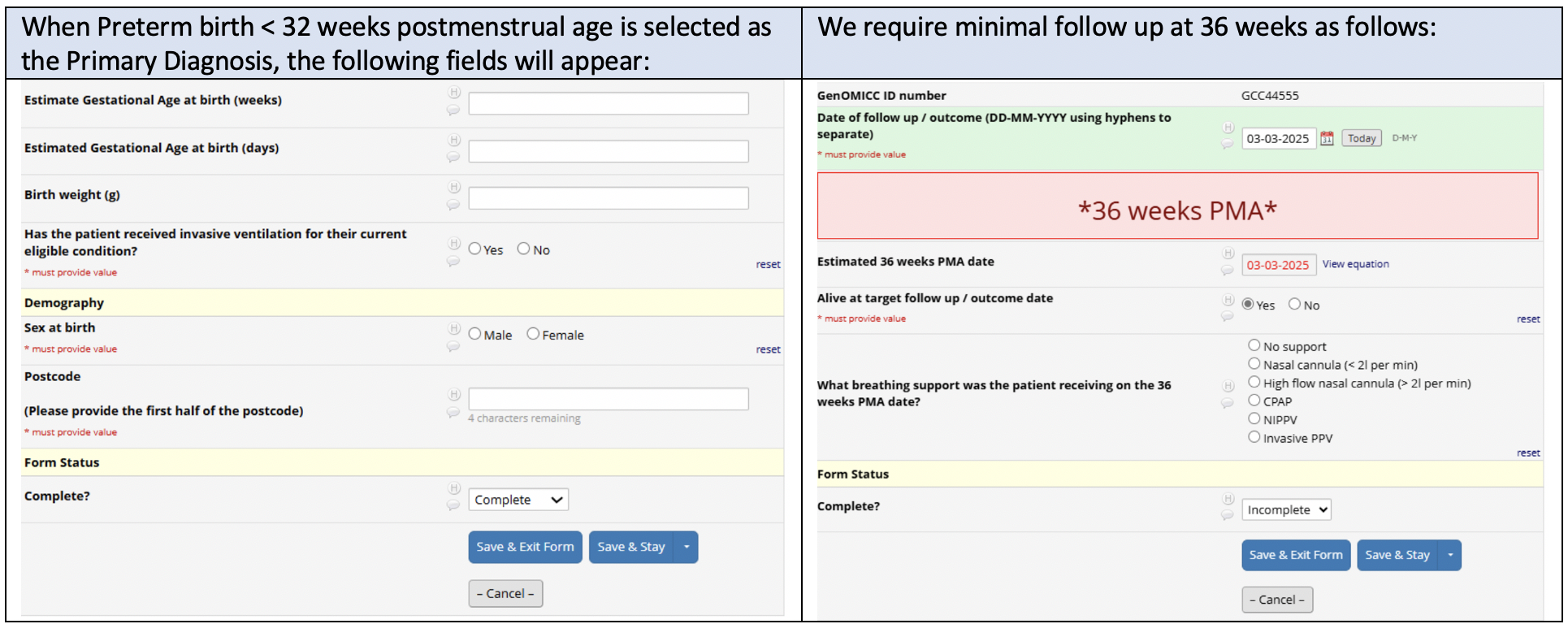
There are only 5 short pages on the preterm eCRF. In addition to some standard checks and collection of personal information, the only other information we need is noted below:
We’re here to help, so please don’t hesitate to contact us with any questions.